cd electron configuration|full electron configuration of platinum : Cebu Atomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa Find & Download Free Graphic Resources for Floral Clipart. 962,000+ Vectors, Stock Photos & PSD files. Free for commercial use High Quality Images.
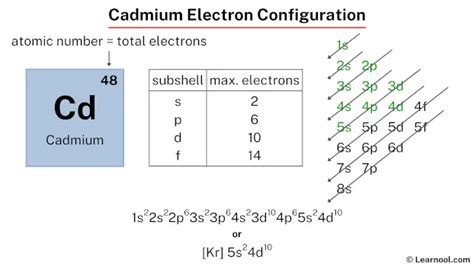
cd electron configuration,The ground-state electron configuration of cadmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. This electron configuration shows that the last shell of cadmium has two electrons and the d-orbital has a total of ten electrons. Therefore, the valence electronsof cadmium are two. The elements that form . Tingnan ang higit pa
The total number of electrons in cadmium is forty-eight. These electrons are arranged according to specific rules in different . Tingnan ang higit pa
Scientist Niels Bohr was the first to give an idea of the atom’s orbit. He provided a model of the atom in 1913. The complete idea of the orbit is given there. The electronsof . Tingnan ang higit paAtomic energy shells are subdivided into sub-energy levels. These sub-energy levels are also called orbital. The most probable . Tingnan ang higit pa Mar 23, 2023 We first need to find the number of electrons for the Cd atom (there are 48 electrons) using the Periodic Table. When we write the configuration, we'll pu .more. To write the.cd electron configuration full electron configuration of platinum Electron Configuration of Cadmium. Cd: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s2 4d 10 or [Kr] 4d 10 5s 2
114 Cd Electron configuration [Kr] 4d 1 0 5s 2 CAS number: 7440-43-9 ChemSpider ID: 22410: ChemSpider is a free chemical structure database Cadmium Electron Configuration: Cadmium is a chemical element which has a chemical symbol Cd. The atomic number of the Cadmium is 48. It is soft, bluish-white metal and is chemically similar to .

First, find electrons of cadmium atom. Periodic table | Image: Learnool. The atomic number of cadmium represents the total number of electrons of cadmium. Since the atomic number of cadmium is 48, the .Cadmium (Cd) has an atomic mass of 48. Find out about its chemical and physical properties, states, energy, electrons, oxidation and more.
To write the electron configuration of an atom, identify the energy level of interest and write the number of electrons in the energy level as its superscript as follows: 1s 2. This is the electron configuration of .
By “building up” from hydrogen, this table can be used to determine the electron configuration for any atom on the periodic table. We will now construct the ground-state .
Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full. . Electron configuration of Cadmium .
The cadmium electron configuration, denoted as [] 5s 2 4d 10 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10, showcases the specific placement of electrons within the atom.This configuration can .2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is the representation of the arrangement of .Cadmium is a chemical element of the periodic table with chemical symbol Cd and atomic number 48 with an atomic weight of 112.414 u and is classed as a transition metal. . Electron configuration chart. 1s 2: 2s 2: 2p 6: 3s 2: 3p 6: 3d 10: 4s 2: 4p 6: 4d 10: 5s 2: Electrons per shell: 2, 8, 18, 18, 2: Valence electrons : 2: Valency electrons : 2:
Comprehensive information for the element Cadmium - Cd is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions. . Electron Configuration: 1s 2 2s 2 p 6 3s 2 p 6 d 10 4s 2 p 6 d 10 5s 2; Electrons per Energy Level: 2,8,18,18,2 Shell Model .
An atom's electron configuration describes the way its electrons fill sublevels when the atom is in its ground state. Atoms seek the most stable electron configuration, so sublevels are half-filled or fully-filled .
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ).
The electron configuration of Cadmium is 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6 4d10 5s2. The chemical element located in group 12 of the periodic table is called cadmium, its atomic number is 48 and the symbol of this element is Cd. It is characterized by being a bluish white metal, heavy and soft, it is normally little abundant . It has high toxicity.
Cadmium. Element 48 of Periodic table is Cadmium with atomic number 48, atomic weight 112.411. Cadmium, symbol Cd, has a Simple Hexagonal structure and Silver color. Cadmium is a Transition Metal element. It is part of group 12 (zinc family). Know everything about Cadmium Facts, Physical Properties, Chemical Properties, Electronic .Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table.. The electron configuration for the first .

Electron Configuration of Cadmium. Cd: 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s2 4d 10 or [Kr] 4d 10 5s 2. Common Isotopes. Cadmium has a total of eight naturally occuring isotopes. These are 106 . Add an electron to the anion electron configuration. For example, the ground state electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. For Cl −, it will be 1s²2s²2p⁶3s²3p⁶. Remove the outermost electrons in the cation, e.g. electron configuration for Mg 2+ will be 1s²2s²2p⁶.
full electron configuration of platinum The third major category of elements arises when the distinguishing electron occupies an f subshell. The first example occurs in the case of the lanthanoids (elements having atomic numbers between 57 and 71).The lanthanoids have the general electron configuration [Kr]4d 10 4f i 5s 2 5p 6 5d 0 or 1 6s 2. where i is a number between 0 and .
The electron configurations and orbital diagrams of these four elements are: The alkali metal sodium (atomic number 11) has one more electron than the neon atom. . (Zn, Cd, Hg, as well as Cu, Ag, and Au in Figure 6.29) are not technically transition elements. Cd: properties of free atoms. Cadmium atoms have 48 electrons and the shell structure is 2.8.18.18.2. The ground state electron configuration of ground state gaseous neutral cadmium is [ Kr ]. 4d10. 5s2 and the term symbol is 1S0. Schematic electronic configuration of cadmium. The Kossel shell structure of cadmium.Zinc (Zn), cadmium (Cd) and mercury (Hg) are last d-elements in their periods. Their outer electron configuration is (n — 1)d 10 ns 2. They form +2 ions only (M +2 ions) which have configuration (n — 1)d 10. As the elements or the ions they form have full filled (n — 1)d orbitals, they are not true transition elements.In this case, the cadmium ion carries a positive charge. Cd – 2e – → Cd 2+. Here, the electron configuration of cadmium ion (Cd 2+) is 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10. This cadmium ion (Cd 2+) has forty-eight protons, sixty-four neutrons, and forty-six electrons. Cadmium ion.
cd electron configuration|full electron configuration of platinum
PH0 · full ground state electron configuration
PH1 · full electron configuration of platinum
PH2 · electron configuration of oganesson
PH3 · electron configuration for every element
PH4 · electron configuration chart
PH5 · electron configuration calculator
PH6 · cd ground state electron configuration
PH7 · abbreviated electron configuration for nitrogen
PH8 · Iba pa